The Unseen World: Why Atoms Remain Invisible to Us
Written on
Chapter 1: The Challenge of Seeing Atoms
The initial reason we cannot observe atoms, as common sense might suggest, relates to their size. If we struggle to see even a microbe, which is comparably as vast as a planet is to a grain of sand when juxtaposed with an atom, how could we ever hope to visualize an atom? Yet, this isn't the primary obstacle; after all, microscopes allow us to view microbes. Could we not simply create a larger microscope?
However, the answer is a resounding no.
No matter how large a microscope could be, it would never enable us to visualize an atom. The underlying reason defies common intuition: we perceive photons.
Essentially, we "see" the effects of photons on our retinas, which our brains then interpret as images. Photons are subatomic particles emitted and absorbed by atoms. Thus, the actual reason for our inability to observe atoms—now and in the future—is rooted in the limitations of our visual apparatus. This holds true even with the most advanced microscopes imaginable, including those yet to be developed.
To clarify, the only subatomic particles we can "see" are photons; however, we only interpret images produced by their interaction with our retina. This implies that even if we could perceive on a subatomic scale, our eyes would remain incapable of detecting electrons, protons, or neutrons. We see photons.
Picture protons, electrons, and neutrons as individuals on a beach launching fireworks—photons—into the sky. Our eyes can only perceive the fireworks, not the people. We see PHOTONS.
Section 1.1: The Hydrogen Atom
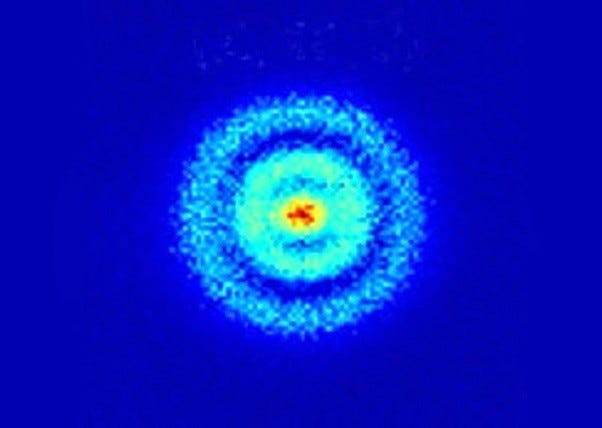
In light of this, let me introduce you to a hydrogen atom!
Surprising, right? You just mentioned that human eyes cannot see an atom. Indeed, and that remains accurate. The "image" of the hydrogen atom was produced through human innovation, translating the invisible into a visual format.
Researchers accomplished this by utilizing a photoionization microscope, which bombarded hydrogen atoms, confined in an electric field, with laser pulses. This process excited the electrons in the hydrogen nucleus's orbit, causing some to be ejected into a detector. After repeated stimulation of the electrons in various ways, they ultimately created this image, representing the closest depiction of a hydrogen atom's electron cloud. In essence, this is not a TRUE image of an atom—we will likely never see one—but it is the most accurate representation we've managed to produce!
If you appreciate my insights, consider supporting my work on Patreon!
Chapter 2: Visualizing the Invisible
This first video, "Why Are Atoms Such a Big Deal and Why Can't We See Them?!", explores the significance of atoms and why they elude our vision.
In the second video, "How Can You See an Atom?", the methods and technologies that attempt to visualize atoms are discussed in depth.